AMolecules arranged in a regular pattern change to an irregular pattern. At the only radioactive.
Molecules separate into their component atoms c.

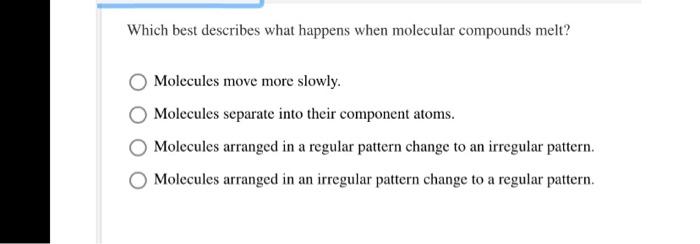
. Which statement best describes what happens when molecular compounds melt. Which of the following best describes what happens when molecular compounds melt. Molecules arranged in a regular pattern changed to an irregular pattern.
Molecules arranged in an irregular pattern change to a regular pattern. Question 2 0f 29 Which best describes what happens when molecular compounds melt. F2 85 K Cl2 239 K Br2 332 K and I2 457 K.
When a molecular compound melts they undergo the process of phase change from solid to liquid therefore molecules arranged in a regular pattern change to an irregular pattern. The molecules then are able to slide around each other liquid phase. What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume.
Molecules arranged in an irregular pattern change to a regular pattern. Molecules separate into their component atoms. A This is a physical change and the molecules move farther apart.
CMolecules move more slowly. Inlace3609 SHOW ANSWER I think the correct answer from the choices listed above is option D. Their melting points are.
What happens when molecular compounds melt. Hope this answers the question. Chemistry As pure elements.
Molecules move more slowly. Which best describes what happens when molecular compounds melt. Molecules arranged in an irregular pattern change to a regular pattern b.
Using periodic table how can i predict the molecular formula of elemental astatine. Describe what happens at the molecular level during melting. DMolecules arranged in an irregular pattern change to a regular pattern.
Molecules move more slowly d. Answers Answer from. All of the halogens are diatomic molecular species.
Which of the following statements best describes what happens when chocolate melts. Molecules arranged in a regular pattern change to an irregular pattern. Which statement best describes what happens when molecular compounds melt.
The em to the trashean chck the rashican to clear all yoarrs on the axes provided label pressure on the honrontal avis from o mb to 760 mb and volume on te vertical from o to 1 mt assign values to axes divisions in such a way that you oceugy almost all h space on both axes now tocate and label the points 90 09 1100 08 1400 0 2 760 0 ad. Chemistry Exam Pre-test 1Which statement best describes what happens when molecular compounds melt. A substance with stronger intermolecular forces will take longer to melt or boil than a substance with weaker intermolecular forces.
Covalent compounds are 2 or more nonmetals bond together. B This is a chemical change and the molecules move farther apart. Molecules begin to move around more and become farther apart.
BMolecules separate into their component atoms. C This is a physical change. 405 21006-Fallzo - SARI Resources Assi Ignment Score.
O Molecules separate into individual atoms. The molecules have enough energy to break the bonds holding them in the ice matrix. The bonds between molecules are broken and the molecules move freely with a lesser degree of bonding with no bonding at all you get a gas rather than a liquid.
Molecules move more slowly and pack tightly together. 1 Which best describes what happens when molecular compounds melt. Molecules arringed in regular pattern change tO an irregular pattern Molecules separate into their component atoms Molecules arranged irregular pattern change t0 rcgular pattern C.
Molecules move more slowly. The melting point of the aspirin was found to be 110-115 degress Celsius.

Solved Which Best Describes What Happens When Molecular Chegg Com

Solved Which Best Describes What Happens When Molecular Chegg Com
Solved Some Phase Changes Require Energy To Occur Whereas Chegg Com
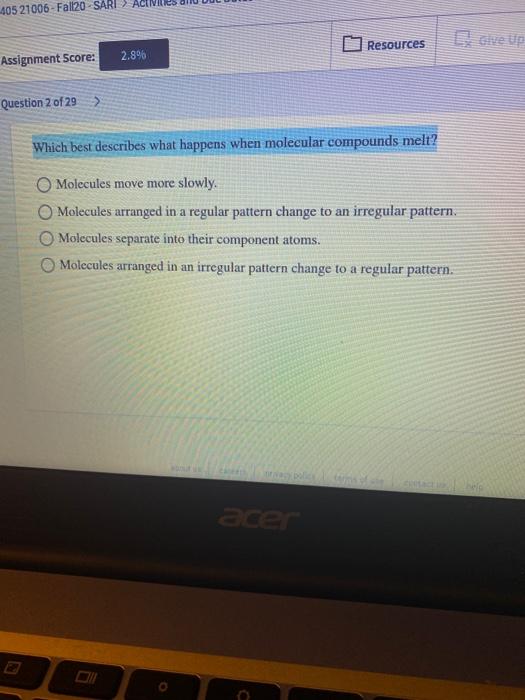
Solved 405 21006 Fall20 Sari Resources 2 8 Assignment Chegg Com

0 Comments